








Our specialty is streamlining supply chains and providing affordable,
sustainable alternatives to many expensive ‘brand name’ products.
Through our valued relationships with our principals and our worldwide
network of suppliers, Silver Fern Chemical, Inc. has the logistics solutions,
expertise, and products to meet the demands of your busy production schedule.
Our innovative market solutions were built with you in mind. We proudly serve the following industries.
With nearly 20-years of experience in bulk chemical distribution and imports,
Silver Fern Chemical provides affordable, personalized, and customizable solutions
for all of your specialty chemical needs. We respond quickly to all inquiries.
We solve specialty and industrial chemical supply dilemmas by being agile. Through our trusted network of alliances, we’re able to source and provide specialty chemicals and ‘equivalent chemical offsets’ at a fraction of the cost. We quickly move products around the globe to provide our customers with faster speed-to-value and to maximize ROI.
We currently serve customers in North America and offer overseas manufacturers an entry point to the North American market. With worldwide distribution centers, we’ll get you the materials you need, when you need them. We’re not just a distributor or an importer, we’re your door-to-door solution.
We believe we are an extension of your business. Our trusted partnerships with the world's leading chemical producers provides our customers with affordable and high-quality products for a variety of applications. We connect you to the materials and services you need to make you the best in your industry.
We are excited to reach this amazing milestone. We could not have achieved this without the hard work, diligence and tenacity of our talented team!!
read moreGreat to get our sales team together and display at the 2023 California Society of Cosmetic Chemists Suppliers Day in Long Beach…
read moreIf you picked up any anti-aging serum and examined the active ingredients, chances are “glycolic acid” would be the top of the list. Glycolic acid has enjoyed cult-like status among skincare formulators for…
read moreSummertime is the season for DIY paint projects. Whether it’s a fresh coat on your backyard fence or a project to spruce up an old dresser, many consumers choose to time their projects…
read moreIt’s no secret that demand for hand sanitizer has skyrocketed since the COVID-19 outbreak started in January. Everyday consumers have seen sanitizer bottles fly off supermarket shelves, and the online retailer Amazon placed…
read moreThe new year is almost upon us, and you know what that means – raw material allocation planning! Chemical industry procurement is uniquely challenging because purchasing agents must be experts in general chemistry,…
read moreBecause of its versatility as an intermediate, Glyoxal is the chemical of choice for a number of manufacturing applications. It is, for instance, a very efficient crosslinker of cellulose in both paper and textile manufacturing. Standard methods of Glyoxal…
read moreThe Going Green and Clean Label trends are now, unquestionably, global pursuits. The quest for natural, non-toxic, environmentally sensitive raw materials will continue to grow as manufacturers and consumers look to reduce the environmental and…
read moreIn March 2019, EPA issued a rule to prohibit the manufacture (including import), processing, and distribution of methylene chloride in all paint and coating removers for consumer use. The EPA goes to some lengths to describe what they mean…
read morePlant-based sources of protein are trending in popularity, both for ordinary dietary supplementation and in the sports nutrition category. While many consumers are interested in protein supplementation, and are drawn to a more plant-centric diet, the texture of plant-protein…
read moreWe deliver products on time to hundreds of valued customers,
from small end users to large Fortune 500 clients, across a diverse range of industries.










We are proud members and supporters of the National Association of Chemical Distributors and The Indis Group.


We manage inventory to meet your demands on short notice. With strategically located distribution centers throughout North America, we will deliver what you need, on time, at the right price!
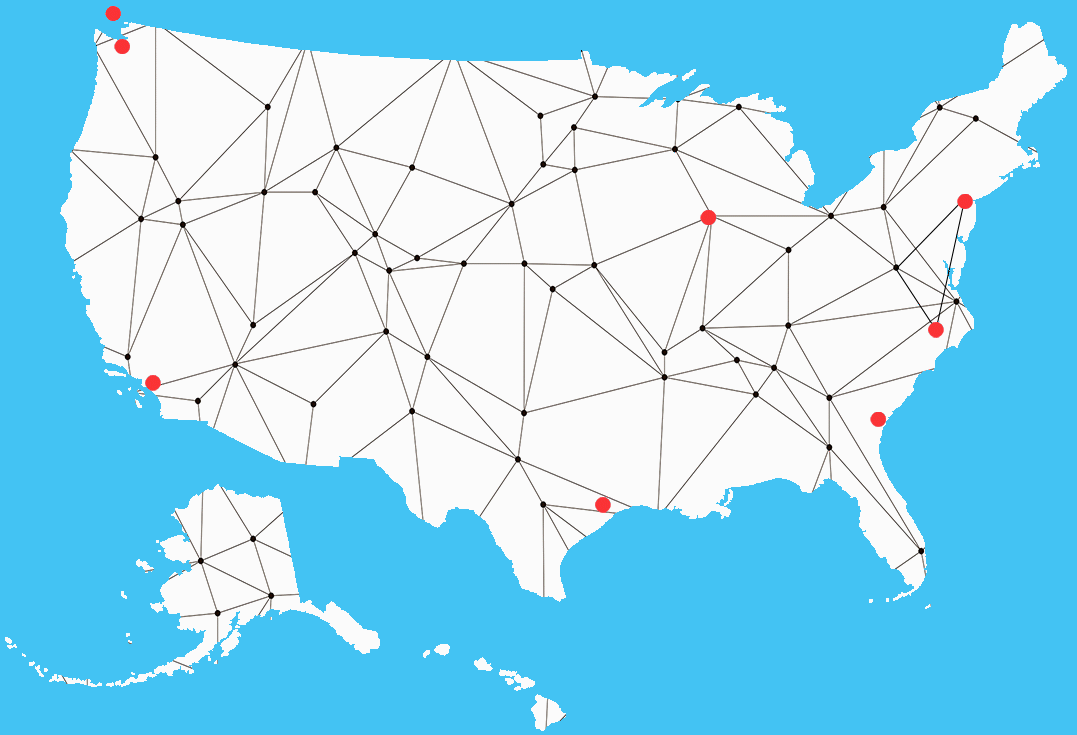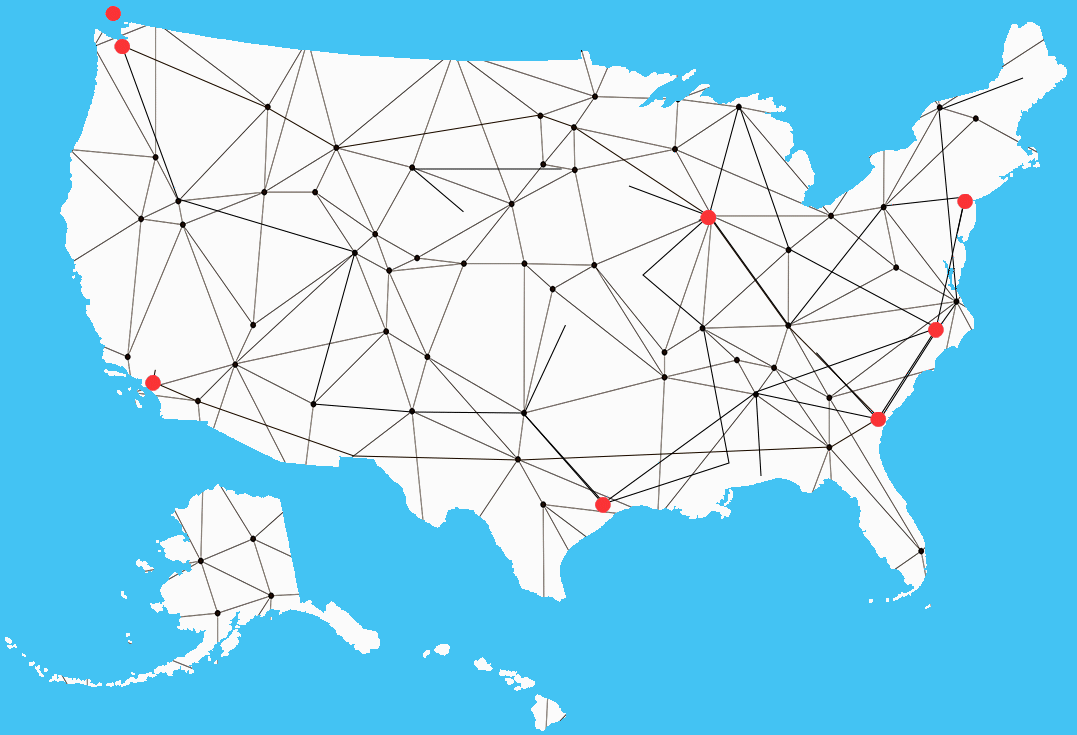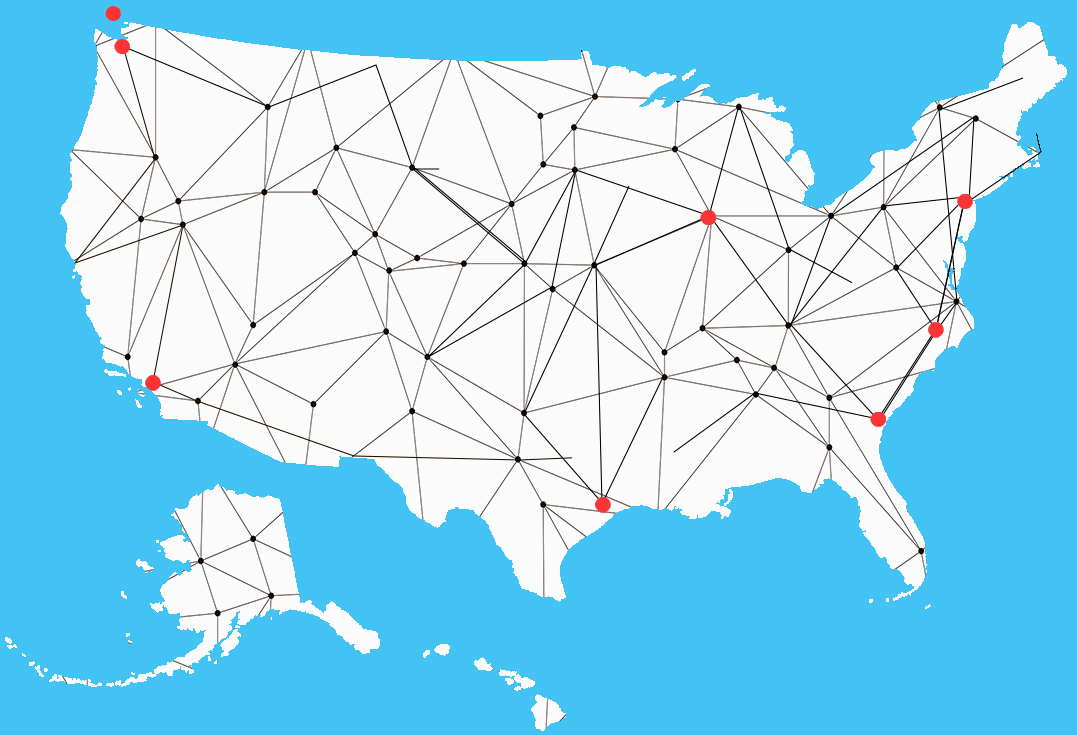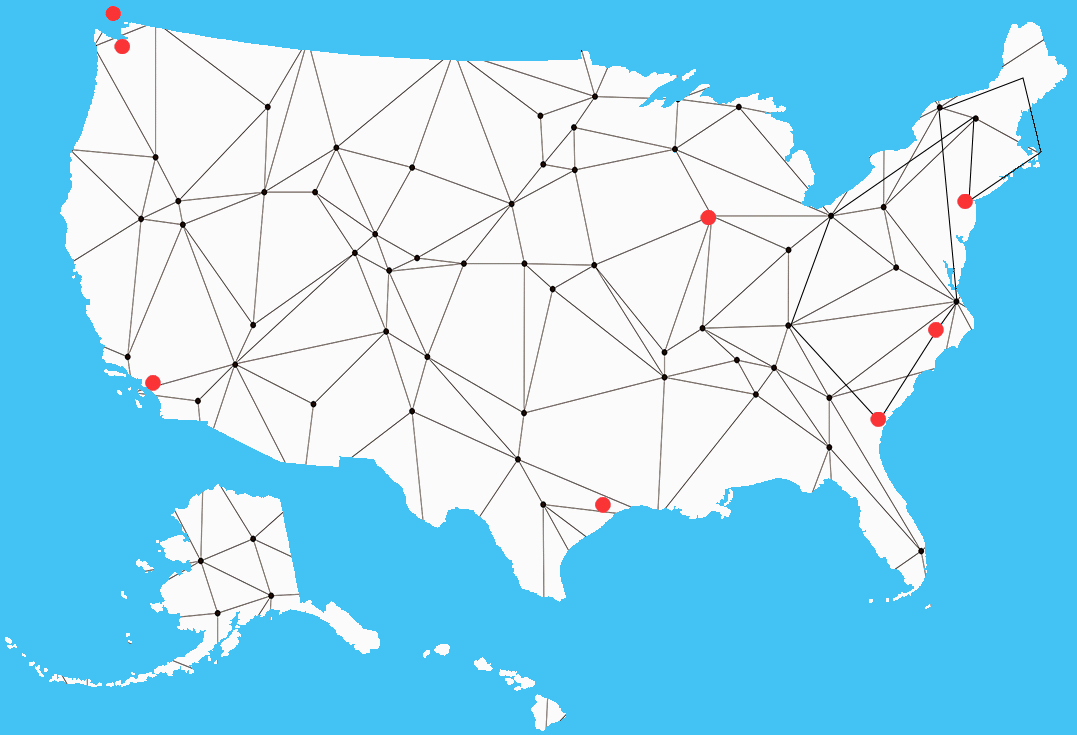
Main Warehouse Locations
No, we sell to businesses only.
Generally not.
Yes!
Yes, we take most major credit cards.
MOQ is usually a 55 gallon drum for liquids and a pallet of bags for solids. We do not sell laboratory sizes.
You may be able to schedule a carrier to pick up your order from one of our warehouses. Please inquire with your salesperson for details.
Yes! We can ship tank trucks, isotanks, and even rail cars to suit your needs.
Yes! Some products are available with extra certifications. Please inquire for details.
Yes. The specifications published on this website are for informational purposes. Ask your account manager if there is something specific you are looking for.